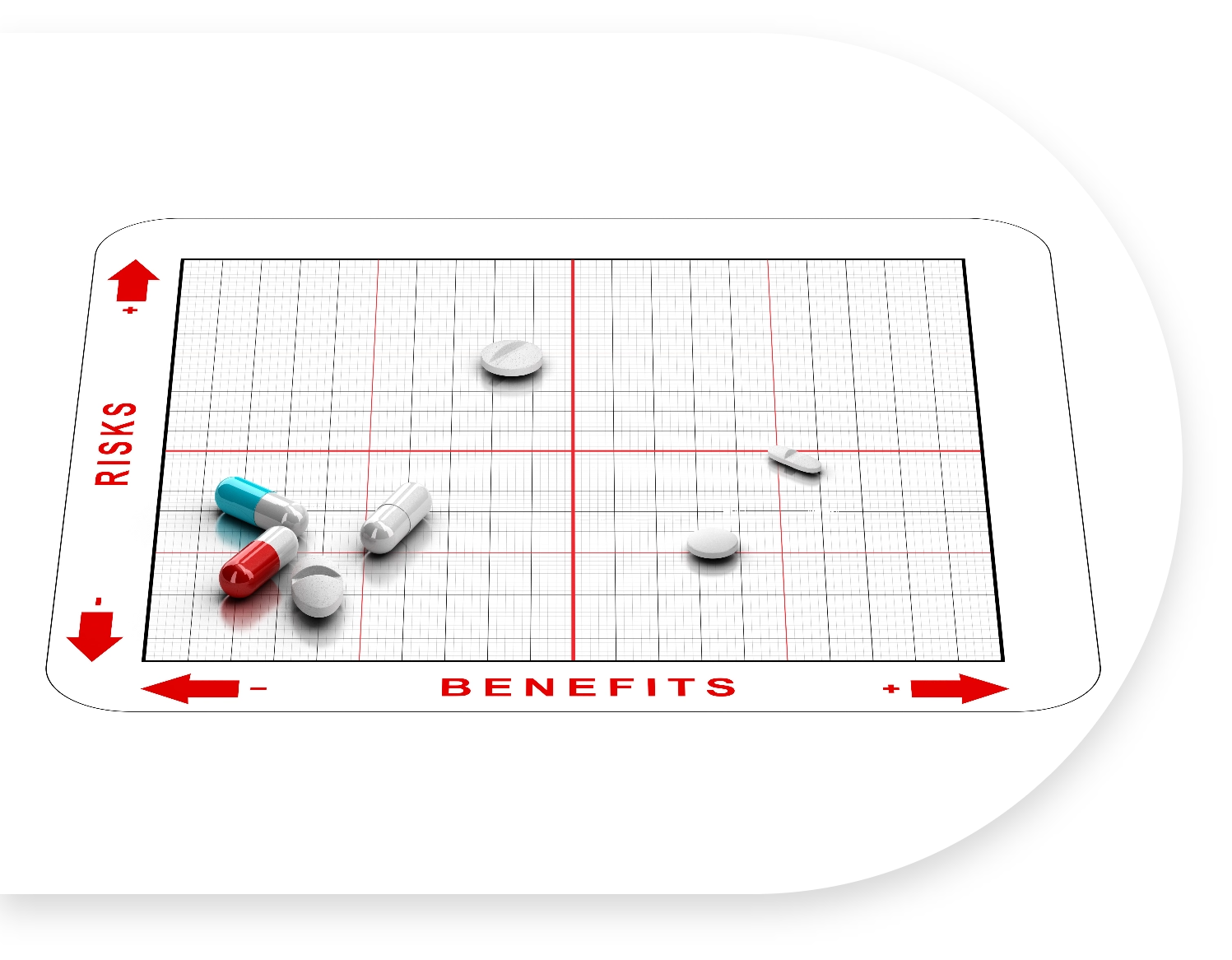
Why Capture Patient Perspectives
QualityMetric's patient-reported outcome (PRO) measures and clinical outcome assessment (COA) services can provide the answers you need to take your product from innovative idea to powerful solution at every phase of your clinical trial. Reach out to us today to learn more about PRO health surveys for your next clinical trial.