NEW COAS IN ONCOLOGY GUIDE
NEW COAS IN ONCOLOGY GUIDE
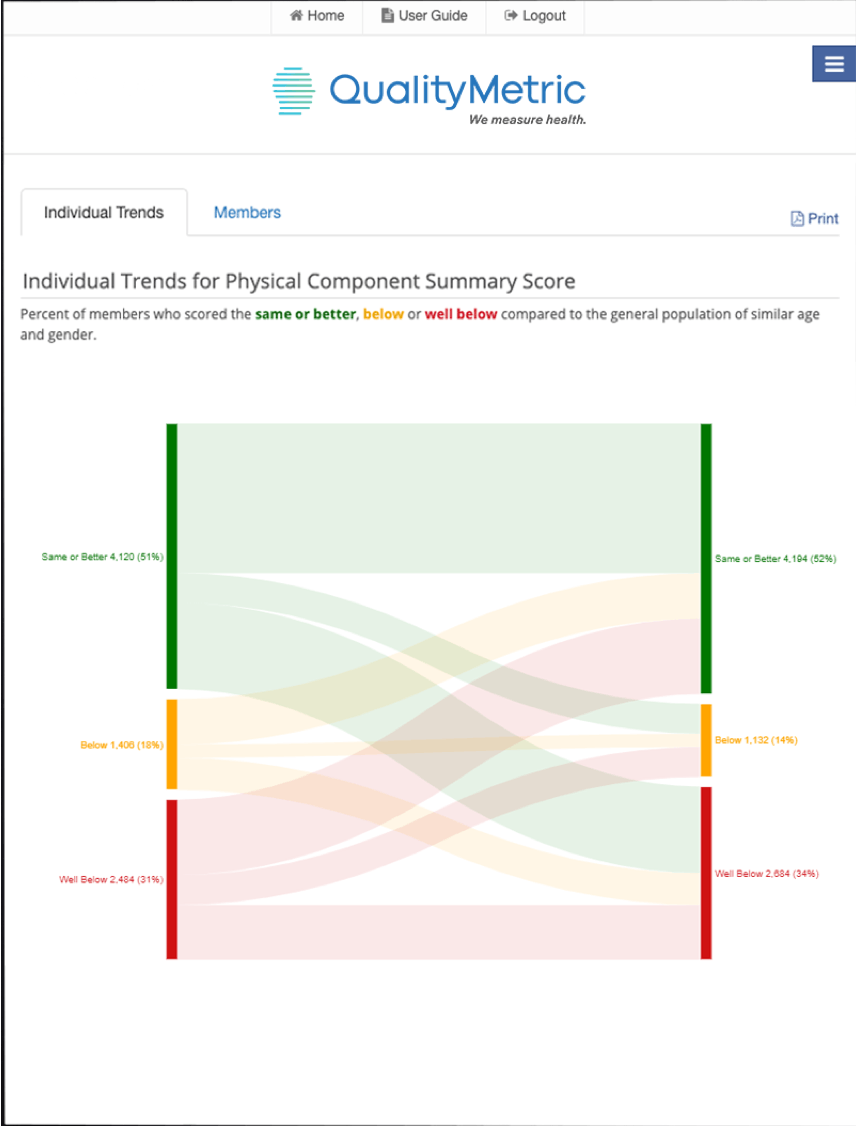
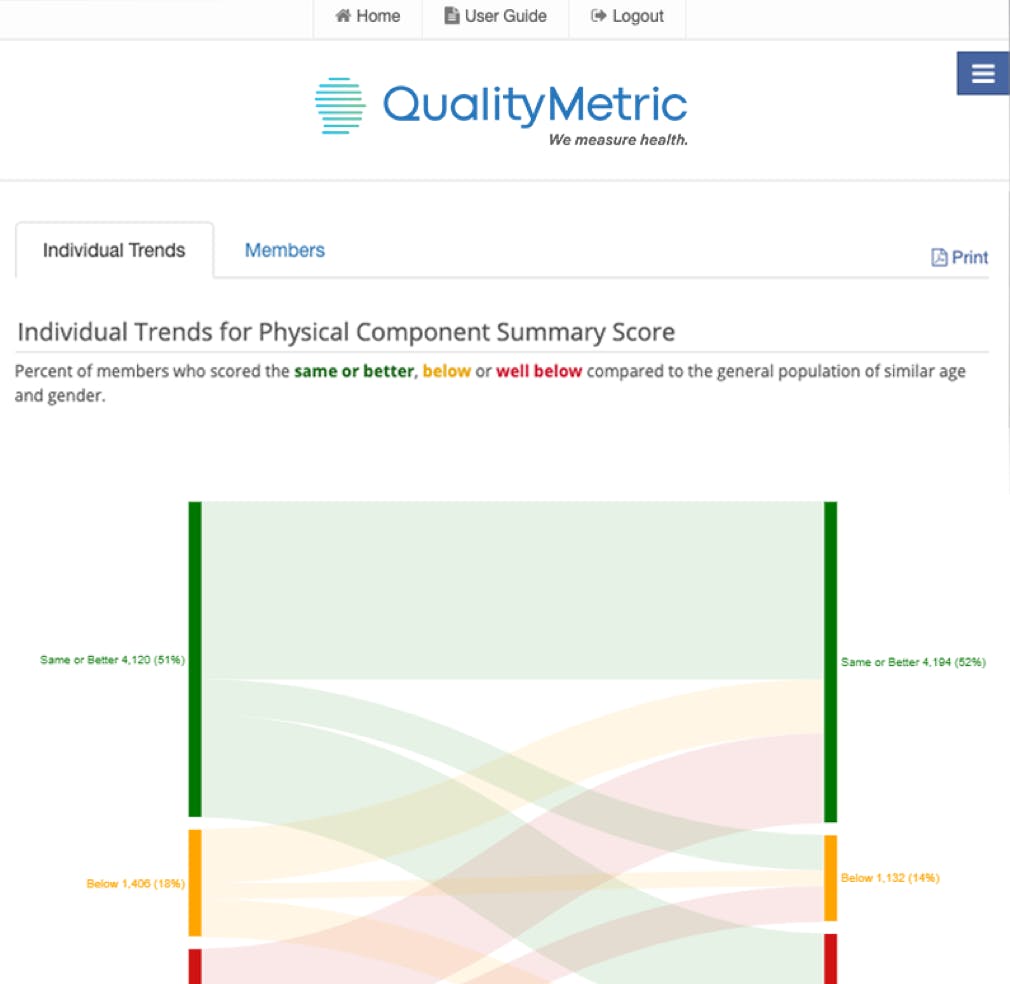
QM COAs can help capture slowed decline in physical functioning and well-being. Learn how to illuminate the impact of cancer treatments and therapies using COA measures in a free oncology-focused guide.
Read the Guide