Clinical Outcome Assessment (COA) Strategy
Understanding how rare diseases and new treatments affect patients
QualityMetric is an expert in rare disease evidence generation. We take an end-to-end approach to build novel instruments and support new label claims for therapies.
Contact us for a review of our capabilities by phone at (800) 572-9394 or by filling out the form below. For Academic organizations click here.
Select the organization that best describes your company:
Survey License Application
Please answer the following questions and we will be in touch regarding your license
Thank you for your request.
QualityMetric is reviewing your application and will get back to you shortly with approval to move forward with a license to administer, collect, score, and interpret the data for your project/study.
Next steps:
If you have started administering the survey, please email a copy of the survey being administered to [email protected]. We will QA the form for any changes that may affect the scoring and interpretation of the data collected. (Fees may apply).
If you are applying for a student license, please email a copy of your student ID to [email protected].
QUALITYMETRIC HAS CREATED MORE THAN 25 PROPRIETARY
INSTRUMENTS AND SUPPORTED 50 LABEL CLAIMS
The Challenges in Rare Disease
Often there is scant evidence and low understanding of rare disease populations. Disease onset, presentation and the patient experience are not well understood. The natural history of the disease and treatment journey with associated short- and long-term outcomes may not be well documented.
Our end-to-end approach to
PRO instrument development / determining fit
of existing PRO Instruments
Our end-to-end approach to
PRO instrument development / determining fit
of existing PRO Instruments
QualityMetric takes an end-to-end approach to helping clients put together a robust evidence generation plan with patient reported outcomes and clinical outcomes assessments that illuminate how diseases impact patients and support new label indications that reflect therapeutic value to patients’ lives. QualityMetric’s approaches include validation of the SF‑36v2, SF‑12v2 and other instruments in a new rare disease, validation of non-QM instruments and design and validation of new PRO instruments including submission of FDA and EMA dossiers to provide evidence of fit for purpose in pursuit of PRO label claims.
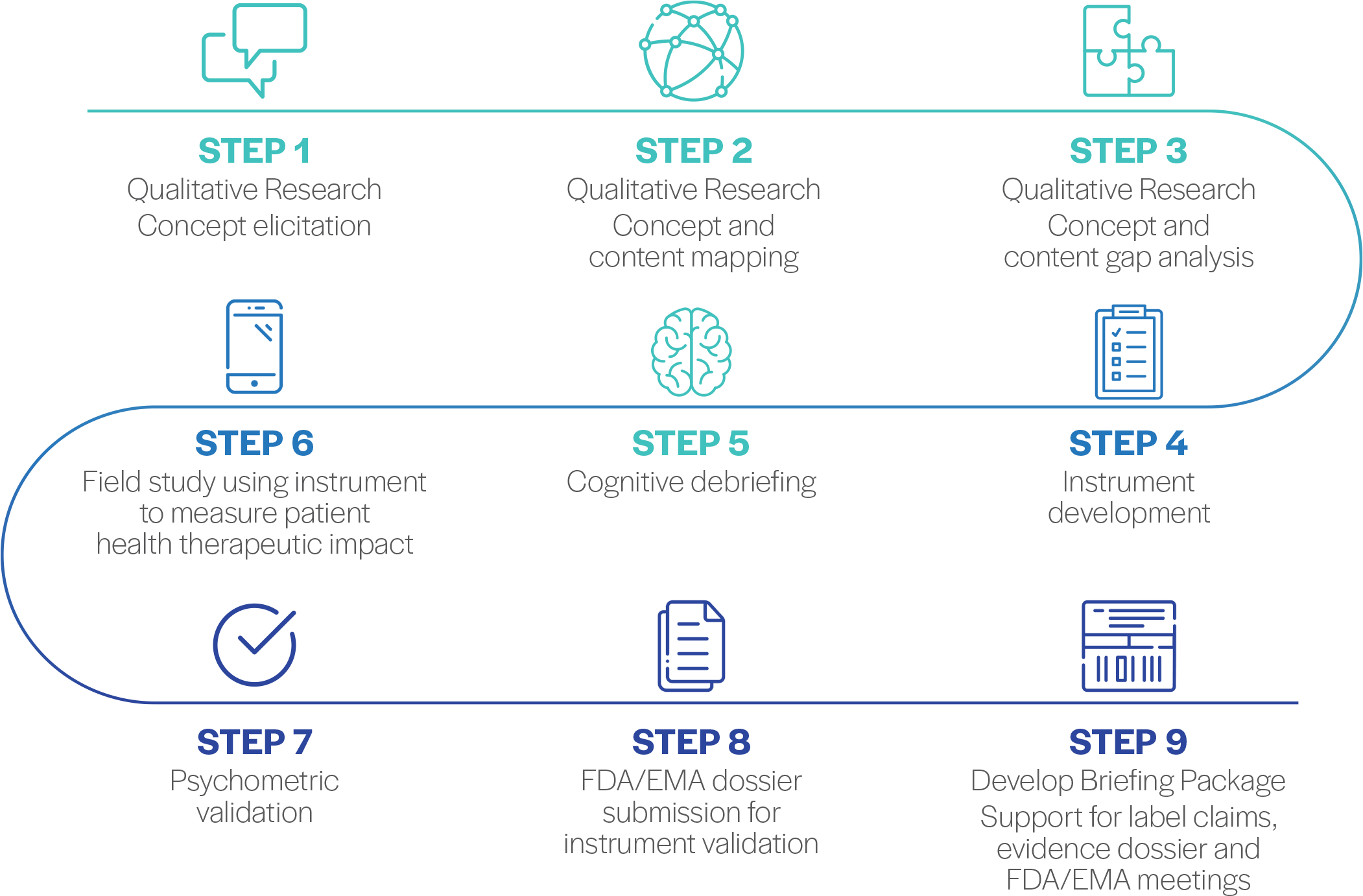
An Expert in Rare Disease
QualityMetric has projects in the following rare diseases:
X-Linked Hypophosphatemia
PRO landscape evaluation and gap analysis
TTR Amyloidosis
New instrument development
Hemophilia
Validation of a physical function scale and
FDA meeting support
Sickle Cell Disease
Presented evidence of physical functioning tool for use in a trial and development and validation of a new daily diary instrument to measure impact of VOC
Bespoke Rare Disease Services
QualityMetric also offers bespoke research services at any phase of research.
TARGETED LITERATURE REVIEWS, SYSTEMATIC LITERATURE REVIEWS
& META-ANALYSES
Provide information related to a rare disease (symptoms, impacts, etc.) and measures used in clinical studies of those diseases.
QUALITATIVE INTERVIEWS
Work alongside patient advocacy groups and clinical professionals to conduct qualitative research studies with clinicians, patients and caregivers.
COA MEASUREMENT STRATEGY
Recommend and develop COAs that are most appropriate for your research purpose.
STUDY DESIGN & INTERPRETATION
Burden of Disease: Compare functional health status against conditions with a significant impact on quality of life to contextualize patient scores.
Treatment Efficacy: Assist your team in determining study endpoints, trend analyses, categories of change and clinical markers to establish benefits of different treatments.
PSYCHOMETRIC VALIDATION
Evaluate psychometric properties of COAs in a particular disease.
REGULATORY SUPPORT
Ensure your COA tools adhere to global regulatory standards, including those of the FDA, EMA and other regulatory agencies worldwide.
PATIENT ADVOCACY
We partner with patient advocacy groups to assist in patient recruitment and sustained engagement.
